Time theft is an irreplaceable concept that directly extends to how light impacts mitochondrial function at the mtDNA level. In mammals, ultraweak photon emissions (UPEs) are generated, relying on circadian timing to optimize energy transformation via the electron transport chain (ETC). Sunlight, rich in red and near-infrared wavelengths (600-1000 nm), penetrates tissues and enhances cytochrome c oxidase (CCO) activity, boosting ATP production and reducing reactive oxygen species (ROS).

This efficient process is disturbed by hypoxia at the mtDNA level, where UPE spectra shift, often narrowing and weakening. This shift mimics the current solar cycle and manifests in humans as chronic diseases. Light stress has historically contributed to significant extinction events on Earth; metabolic stress from inadequate light adaptation has plagued numerous species.
Artificial light sources, particularly LEDs, lack the full spectrum of sunlight, especially in the red and infrared wavelengths, and emit high levels of blue light (400-500 nm). Blue light inhibits CCO activity by increasing ROS, which leads to hypoxia and mitochondrial heteroplasmy.

This disruption ultimately “subtracts time” from our lifespans, accelerating cellular aging through disrupted circadian rhythms (via melanopsin in the retina/SCN) and impairing mitochondrial UPE production. Over time, these effects manifest as chronic diseases.
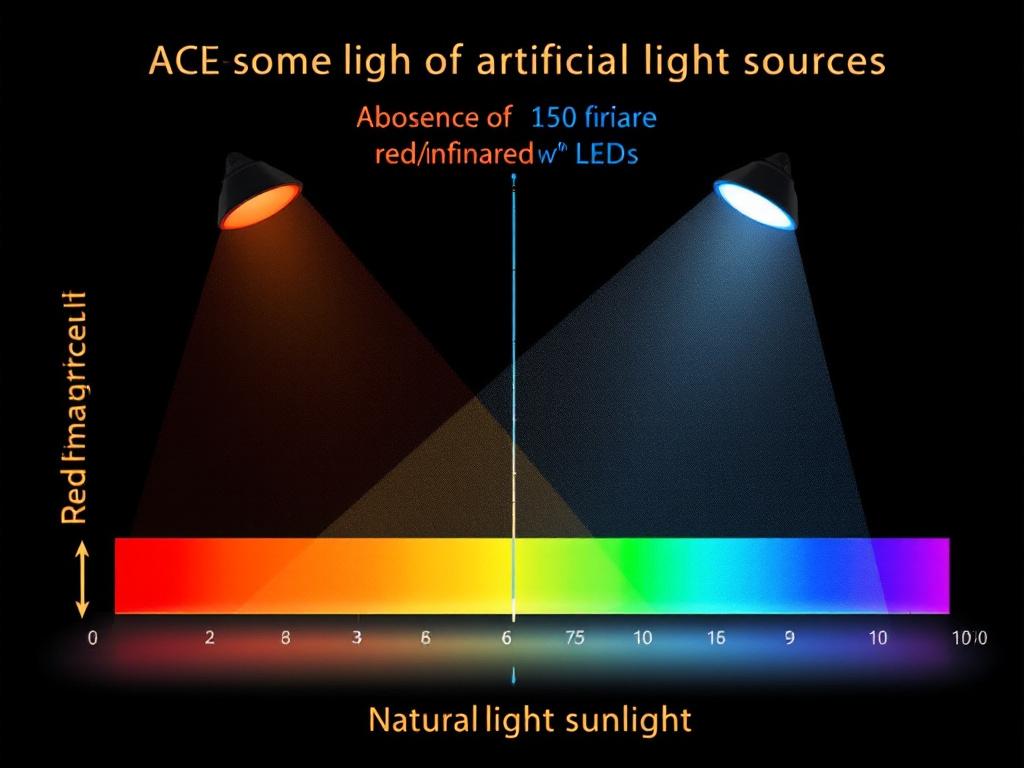
Understanding the detrimental impacts of light on our biology, especially concerning artificial sources, is crucial for our health. The negative effects of blue light continue to add significant stress on mitochondrial function and circadian rhythms.
In conclusion, the interplay between light and mitochondrial function is a vital relationship that requires greater understanding and recognition to combat the chronic diseases proliferating in our modern world.